Pfizer - HAN
Jump To:
Overview
Interested in the Pfizer-BioNTech COVID 19 Vaccine?
This page contains information for the Pfizer-BioNTech COVID-19 vaccine.
Please check in frequently as information will be posted as it becomes available.
Please check in frequently as information will be posted as it becomes available.
Resources and Information for Pfizer-BioNTech Vaccine
FDA Website for Pfizer-BioNTech COVID-19 Vaccine Information
MMWR: The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine — United States, December 2020 - posted 12/13/2020
What Clinicians Need to Know About the Pfizer-BioNTech COVID-19 Vaccine - posted 12/13/2020
CDC Pfizer-BioNTech Standing Orders - posted 12/16/2020
CDC Pfizer-BioNTech Vaccine Preparation Information - posted 12/16/2020
CDC Pfizer-BioNTech COVID 19 Pre-Vaccination Form - posted 12/16/2020
FDA Website for Pfizer-BioNTech COVID-19 Vaccine Information
MMWR: The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine — United States, December 2020 - posted 12/13/2020
What Clinicians Need to Know About the Pfizer-BioNTech COVID-19 Vaccine - posted 12/13/2020
CDC Pfizer-BioNTech Standing Orders - posted 12/16/2020
CDC Pfizer-BioNTech Vaccine Preparation Information - posted 12/16/2020
CDC Pfizer-BioNTech COVID 19 Pre-Vaccination Form - posted 12/16/2020
Vaccinating Persons with History of Allergies
CDC has issued an MMWR on anaphylaxis reactions for the Pfizer-BioNtech vaccine. 21 cases of anaphylaxis are reported of a total of 1,893,360 vaccine doses administered. 17 of these 21 had a history of allergies or allergic reaction and 7 of these had a history of anaphylaxis. 71% of reported anaphylaxis episodes occurred within the first 15 minutes of vaccination. Read the full report here.
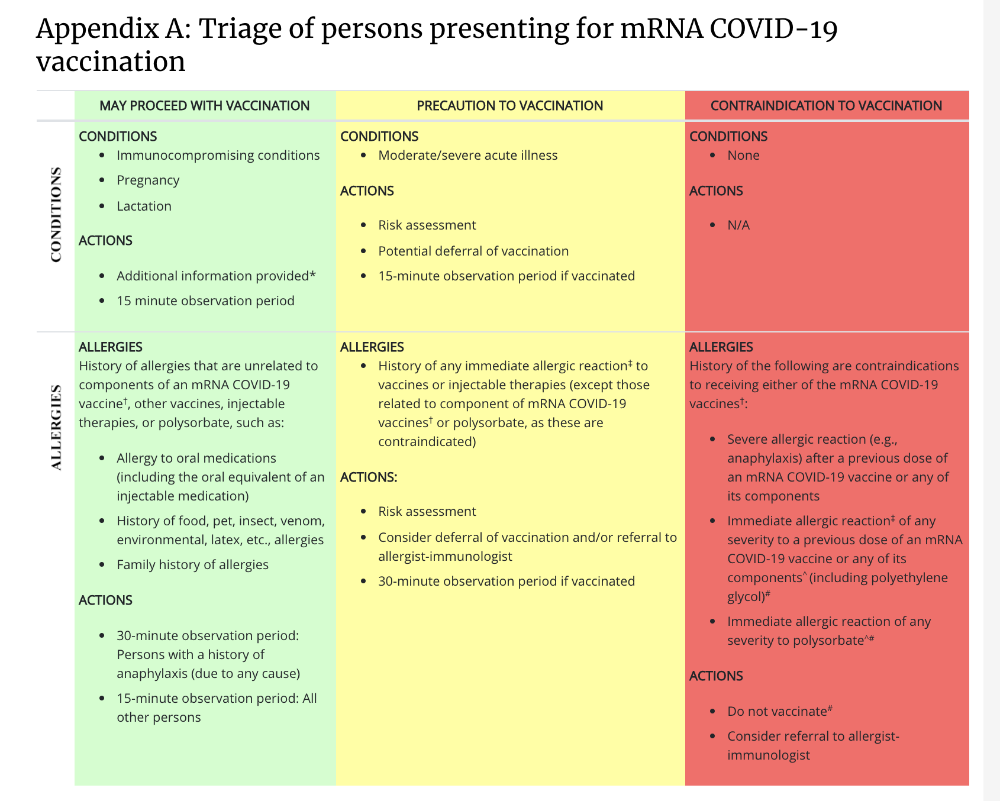
Find full considerations from CDC here including detailed updated information on clinical considerations. Guidelines were updated by CDC on 2/10/21 - including clarifications on contraindications and precautions, information on delayed injection-site reactions and updated quarantine guidance for people who have completed vaccination.
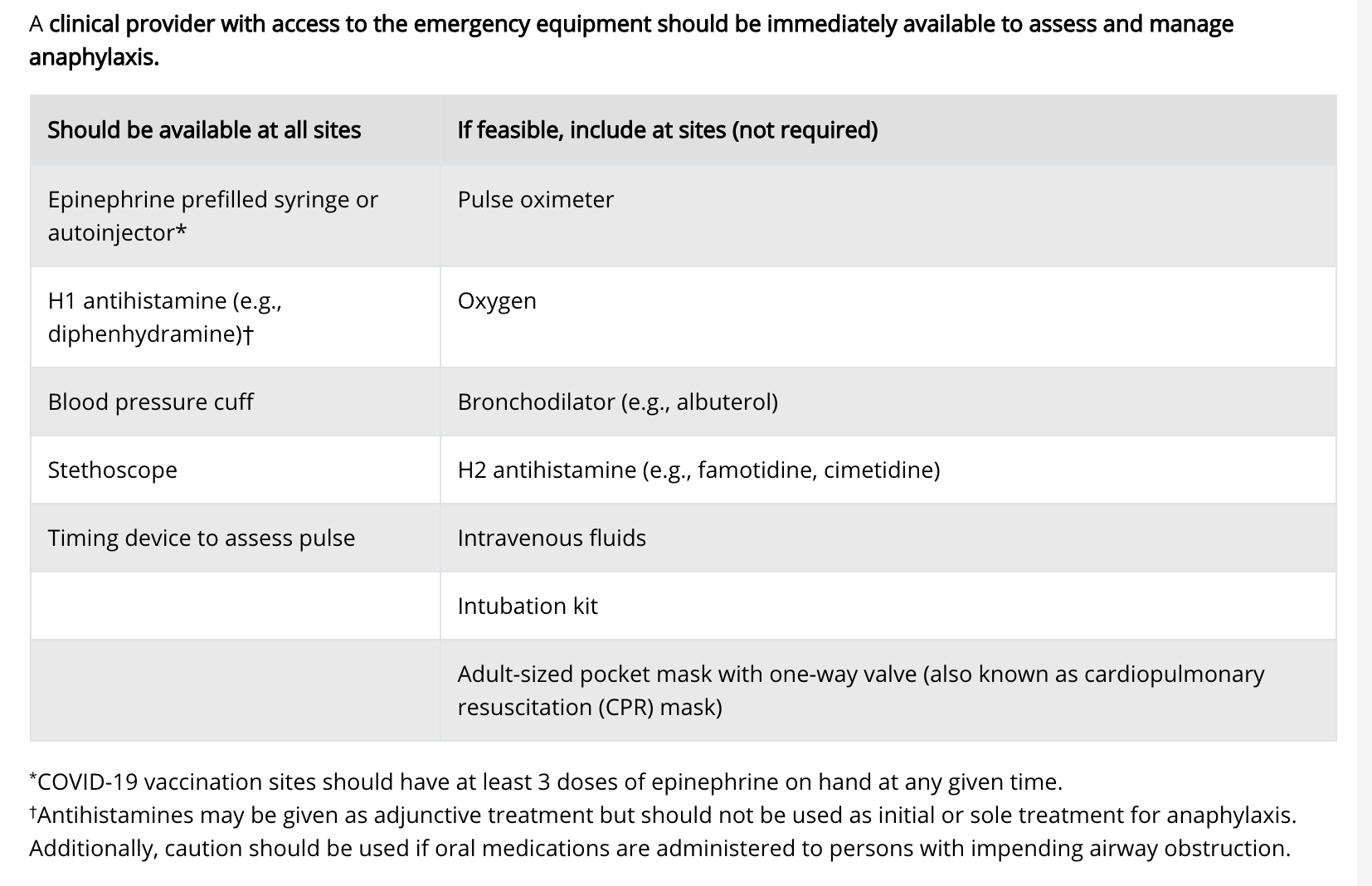

Find full considerations from CDC here including detailed updated information on clinical considerations. Guidelines were updated by CDC on 2/10/21 - including clarifications on contraindications and precautions, information on delayed injection-site reactions and updated quarantine guidance for people who have completed vaccination.

Announcements
5/21/21: Pfizer has received EUA authorization for extended storage of undiluted vials in the refrigerator.
Thawed Vials Before Dilution Thawed Under Refrigeration: Thaw and then store undiluted vials in the refrigerator [2ºC to 8ºC (35ºF to 46ºF)] for up to 1 month. A carton of 25 vials or 195 vials may take up to 2 or 3 hours, respectively, to thaw in the refrigerator, whereas a fewer number of vials will thaw in less time.
NEW: Product Information Guide (issued April 28, 2021)
NEW: Ancillary kit syringe and needle deficiency reporting
NEW COVID-19 Vaccine Provider Videos
Race/Ethnicity Reporting Updates (2/16/21)
Quick Provider Ordering Tips (2/16/21)
VaccineFinder Inventory Reporting in 3 Easy Steps (2/16/21)
Instructions on signing up for ZocDoc (2/8/2021)
CDPH has compiled a provider toolkit which can be used to engage patients and employees eligible for vaccine. Find here under the "toolkits" tab.
NEW CDPH Provider Update - issued Jan 28, 2021
CDC has issued an MMWR on anaphylaxis reactions for the Pfizer-BioNtech vaccine. 21 cases of anaphylaxis are reported of a total of 1,893,360 vaccine doses administered. 17 of these 21 had a history of allergies or allergic reaction and 7 of these had a history of anaphylaxis. 71% of reported anaphylaxis episodes occurred within the first 15 minutes of vaccination. Read the full report here.
Covid-19 Vaccination Chicago Provider Letter from CDPH - posted 12/18/2020
See the CDC site for updated reporting on total administered vaccine doses in the USA.
Thawed Vials Before Dilution Thawed Under Refrigeration: Thaw and then store undiluted vials in the refrigerator [2ºC to 8ºC (35ºF to 46ºF)] for up to 1 month. A carton of 25 vials or 195 vials may take up to 2 or 3 hours, respectively, to thaw in the refrigerator, whereas a fewer number of vials will thaw in less time.
NEW: Product Information Guide (issued April 28, 2021)
NEW: Ancillary kit syringe and needle deficiency reporting
NEW COVID-19 Vaccine Provider Videos
Race/Ethnicity Reporting Updates (2/16/21)
Quick Provider Ordering Tips (2/16/21)
VaccineFinder Inventory Reporting in 3 Easy Steps (2/16/21)
Instructions on signing up for ZocDoc (2/8/2021)
CDPH has compiled a provider toolkit which can be used to engage patients and employees eligible for vaccine. Find here under the "toolkits" tab.
NEW CDPH Provider Update - issued Jan 28, 2021
CDC has issued an MMWR on anaphylaxis reactions for the Pfizer-BioNtech vaccine. 21 cases of anaphylaxis are reported of a total of 1,893,360 vaccine doses administered. 17 of these 21 had a history of allergies or allergic reaction and 7 of these had a history of anaphylaxis. 71% of reported anaphylaxis episodes occurred within the first 15 minutes of vaccination. Read the full report here.
Covid-19 Vaccination Chicago Provider Letter from CDPH - posted 12/18/2020
See the CDC site for updated reporting on total administered vaccine doses in the USA.
Storage and Handling
The following resources are available to assist providers in storing and handling of the Pfizer-BioNTech COVID-19 vaccine.
Dry Ice Saftey Healthcare
Pfizer BUD Tracking Labels
Pfizer Storage Label
Pfizer Storage Summary
Pfizer TempLog ULTRACOLDStorage Celsius
Pfizer TempLog ULTRACOLDStorage Fahrenheit
Vaccine Expiration Date Tracker
Dry Ice Saftey Healthcare
Pfizer BUD Tracking Labels
Pfizer Storage Label
Pfizer Storage Summary
Pfizer TempLog ULTRACOLDStorage Celsius
Pfizer TempLog ULTRACOLDStorage Fahrenheit
Vaccine Expiration Date Tracker
Pfizer Contacts
COVID-19 Vaccine Contact Information
Please direct all questions to COVID19vaccine@cityofchicago.org
For Information On The Vaccine Please Go To:
www.chicago.gov/covidvax
Please visit the Illinois Department of Public Health I-CARE website for information on enrolling a facility or individual in I-CARE: https://www.dph.illinois.gov/topics-services/prevention-wellness/immunization/icare
