Meningococcal Disease - HAN
Jump To:
Overview
Meningococcal Disease (N.meningitidis)
Neisseria meningitidis is a leading cause of bacterial meningitis and sepsis in the United States. Invasive infection can cause meningitis or meningococcemia (meningococcal sepsis) which can be severe and potentially fatal. Meningococcal Meningitis is when the bacteria infect the protective membranes covering their brain and spinal cord causing swelling. Meningococcemia occurs when the bacteria enter the bloodstream and multiplies in the walls of the blood vessels causing septic shock. Other serious sequelae include hearing loss, neurologic disability, and amputation secondary to limb ischemia.
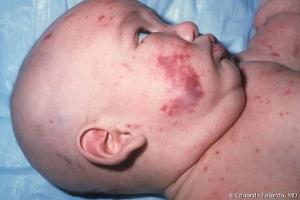
Symptoms
-
Fever/chills
-
Headache
-
Nausea/Vomiting
-
Stiff neck
-
Altered mental status
-
Cold hands and feet
-
Diarrhea
-
Photophobia
-
Dark purplish rash (maculopapular, petechial, or in later stages purpuric)
Note: Photo credit: Redbook 2015
Prophylaxis for High-Risk Contacts and People with Invasive Meningococcal Disease
Exposed persons should receive antibiotic prophylaxis to prevent secondary cases regardless of whether an outbreak is suspected.
TABLE. Recommended chemoprophylaxis regimens for protection against meningococcal disease — Advisory Committee on Immunization Practices (ACIP), United States, 2012
Drug |
Age group |
Dosage |
Duration and route of administration* |
|---|---|---|---|
Rifampin† |
Children aged <1 mo |
5 mg/kg orally, every 12 hrs |
2 days |
Rifampin† |
Children aged ≥1 mo |
15-20 mg/kg orally, every 12 hrs (maximum 600 mg) |
2 days |
Ciprofloxacin |
≥ 1 mo |
20 mg/kg orally(maximum 500mg) |
Single dose |
Ceftriaxone |
<15 yrs |
125 mg intramuscularly |
Single IM dose (To decrease pain dilute with 1% lidocaine) |
Ceftriaxone |
≥15 yrs |
250 mg intramuscularly |
Single IM dose (To decrease pain dilute with 1 % lidocaine) |
† Rifampin is not recommended for pregnant women because the drug is teratogenic in laboratory animals. Because the reliability of oral contraceptives might be affected by rifampin therapy, consideration should be given to using alternative contraceptive measures while rifampin is being administered. Source: Red Book (2018-2021) Meningococcal Infections: Table 3.42. Recommended chemoprophylaxis regimens for high-risk contacts and people with invasive meningococcal disease. |
Clinical Reporting Guidelines
1
Collect samples of blood and CSF if Neisseria meningitis is suspected. If Neisseria meningitidis is identified by culture, send isolate to IDPH laboratory for serogrouping and molecular typing. If no isolate is available send specimens to IDPH. (e.g. Gram-negative diplococci on gram stain and/or positive PCR with negative culture.)
IDPH Laboratory
2121 W. Taylor Street
Chicago, IL 60612
2
Report suspect, probable, or confirmed cases of Meningococcal Disease within 24 hours to the CDPH Provider Hotline (312) 743-9000 during business hours (Monday - Friday 8:00 am – 4:00 pm) or 311 outside of business hours and enter the case in the I-NEDSS Web Portal. Register for a portal account here I-NEDSS Web Portal.
(Gram stain of CSF showing gram-negative diplococci strongly suggests meningococcal meningitis)
3
Complete the IDPH Communicable Diseases Laboratory Test Requisition Form and send it with the clinical materials. An authorization code is not required to submit these specimens to the public health lab.
4
Ship specimens or isolates to:
Illinois Department of Public Health Laboratory
2121 W. Taylor Street
Chicago, IL. 60612
Once received, IDPH confirms the identification and determines the serogroup of N. meningitidis isolates. Selected isolates are forwarded to CDC laboratories for additional molecular characterization.
CDPH Webinars On Meningococcal Disease
Third Webinar: Meningococcal Disease and HIV: Is Your Patient at Risk?
Webinar: April 7, 2017; Link to webinar here.
Chicago serogroup meningococcal disease outbreak
Meningococcal disease in HIV Positive persons
Vaccine recommendations and outbreak information
Ramona Bhatia, MD, HIV/STI Program
Related Material: Recommendations for the User of Meningococcal Conjugate Vaccines in HIV-Infected Persons - ACIP, 2016
Webinar recording courtesy of MATEC.
Second Webinar: Meningococcal Outbreak Response: Vaccination Recommendations for Men who have Sex with Men (MSM) in Chicago
Webinar: August 14, 2015; Link to webinar here. Presentations links below.
Summary of the meningococcal outbreak among men who have sex with men (MSM) in the Chicago Metropolitan Area and updates on invasive meningococcal disease among MSM nationally
Ongoing meningococcal vaccination response in Chicago
2 months into the outbreak – anticipating 2nd dose for those living with HIV
How to order and manage 317 meningococcal vaccine
Client Vaccine Uptake Survey and Provider Survey results
Discussion of challenges and successes in achieving higher vaccination coverage rates
Webinar recording courtesy of EverThrive Illinois.
Presentations by:
Sarah Kemble, MD, Medical Director, Communicable Diseases, Chicago Department of Public Health, and Temitope Folaranmi, MBChB, MPH, MPP, EIS Officer, Centers for Disease Control and Prevention
Meningococcal Outbreak Response Partners Meeting, held 11/17/2015
Summary of outbreak and response
Partners Survey results
Discussion and Feedback
Presentation slides available here.
First Webinar: Presentation on vaccine recommendations for Men who have Sex with Men (MSM) in Chicago now available.
In this webinar for medical providers who care for MSM, topics include:
Summary of the meningococcal outbreak among men who have sex with men (MSM) in the Chicago Metropolitan Area
Review of meningococcal vaccination efforts including geographic and demographic distribution of federally-funded vaccine
Information on how to order 317 meningococcal vaccine for Chicago providers
Presentation Information
Presentor: Sarah Kemble, MD, Medical Director, Communicable Diseases, Chicago Department of Public Health
Webinar recording courtesy of EverThrive Illinois. Link below will take you to another site.
Case Definitions
Suspected Case
-
A clinically compatible case and gram-negative diplococcic in any sterile fluid, such as CSF, synovial fluid, or scraping from a petechial or purpuric lesion
-
Clinical purpura fulminans without a positive culture
Probable Case
A clinically compatible case with EITHER a positive result of antigen test OR immunohistochemistry of formalin-fixed tissue
Confirmed Case
A Clinically compatible case and isolation of Neisseria meningitidis from a usually sterile site, for example:
Blood
Cerebrospinal fluid (CSF)
Synovial Fluid
Pleural fluid
Pericardial fluid
Isolation from skin scraping of petechial or purpuric lesions
OR
Detection of N meningitidis-specific nucleic acid in a specimen obtained from a normally sterile body site (e.g., blood or CSF), using a validated polymerase chain reaction (PCR) assay.
Source: Red Book (2018-2021 P.553) Meningococcal Infections: Table 3.40. Surveillance Case Definitions for Invasive Meningococcal Disease
Please include the following data elements when reporting cases:
Patient Name, DOB, Address
Gram Stain Results
Include specimen source
Culture results from CSF Blood (if available)
CSF cell count, Protein, Glucose
Peripheral White Blood Cell (WBC)
Date of Symptom Onset
Emergency Contact
Location 7-10 days prior to symptom onset
* The incubation period for Meningococcal Disease can range from 2 to 10 days.
Meningococcal Resources
Resources
Title |
Source |
Type |
|---|---|---|
CDC |
Website |
|
CDC |
Website |
|
CDC |
Website |
|
CDC |
Website |
About Meningococcal Disease
Title |
Source |
Type |
|---|---|---|
CDC |
Website |
|
Best Practices for Use of PCR for Diagnosing Neisseria meningitidis |
CDC |
Website |
CDC |
||
IDPH |
Website |
|
IDPH |
||
IMD |
||
Enhanced Meningococcal |
Previous Meningococcal Han Alerts
CDPH Information and Previous HAN Alerts regarding Invasive Meningococcal Disease
CDC: Patients Receiving Eculizumab (Soliris®) at High Risk for Invasive Meningococcal Disease
Invasive Meningococcal Disease in Men Who Have Sex with Men HAN #1
Invasive Meningococcal Disease in Men Who Have Sex with Men HAN #3
Invasive Meningococcal Disease in Men Who Have Sex with Men HAN #4
Invasive Meningococcal Disease in Men Who Have Sex with Men HAN #5
Meningococcal Contacts
For Clinical Questions Contact:
Stephanie Black, MD 312-746-6034
For Other Questions Contact:
For questions during non-business hours call 311. (312-744-5000 if outside the City of Chicago.)
