Therapeutics and Prophylaxis - HAN
Jump To:
New COVID-19 Therapeutics Announcements
- March 8, 2024: USG-procured, EUA-labeled Paxlovid is no longer authorized for use after March 8, 2024. All EUA-labeled Paxlovid must be returned or disposed. Only NDA-labeled Paxlovid may be dispensed
- January 29, 2024: FDA Revises the Emergency Use Authorization Letter for Paxlovid
- January, 2024: The PAXCESS Patient Support Program Provides Costs Saving Options for Individuals in Need of Paxlovid
- December 20, 2023: Sunsetting the U.S. Government COVID-19 Therapeutics Distribution Program: A Transition Guide
Overview of COVID-19 Therapeutics and Prophylaxis for Non-Hospitalized Patients
The resources below provide:
- Interim guidance on outpatient treatment decisions for patients at risk of developing severe COVID-19.
- Comparison table of treatment options.
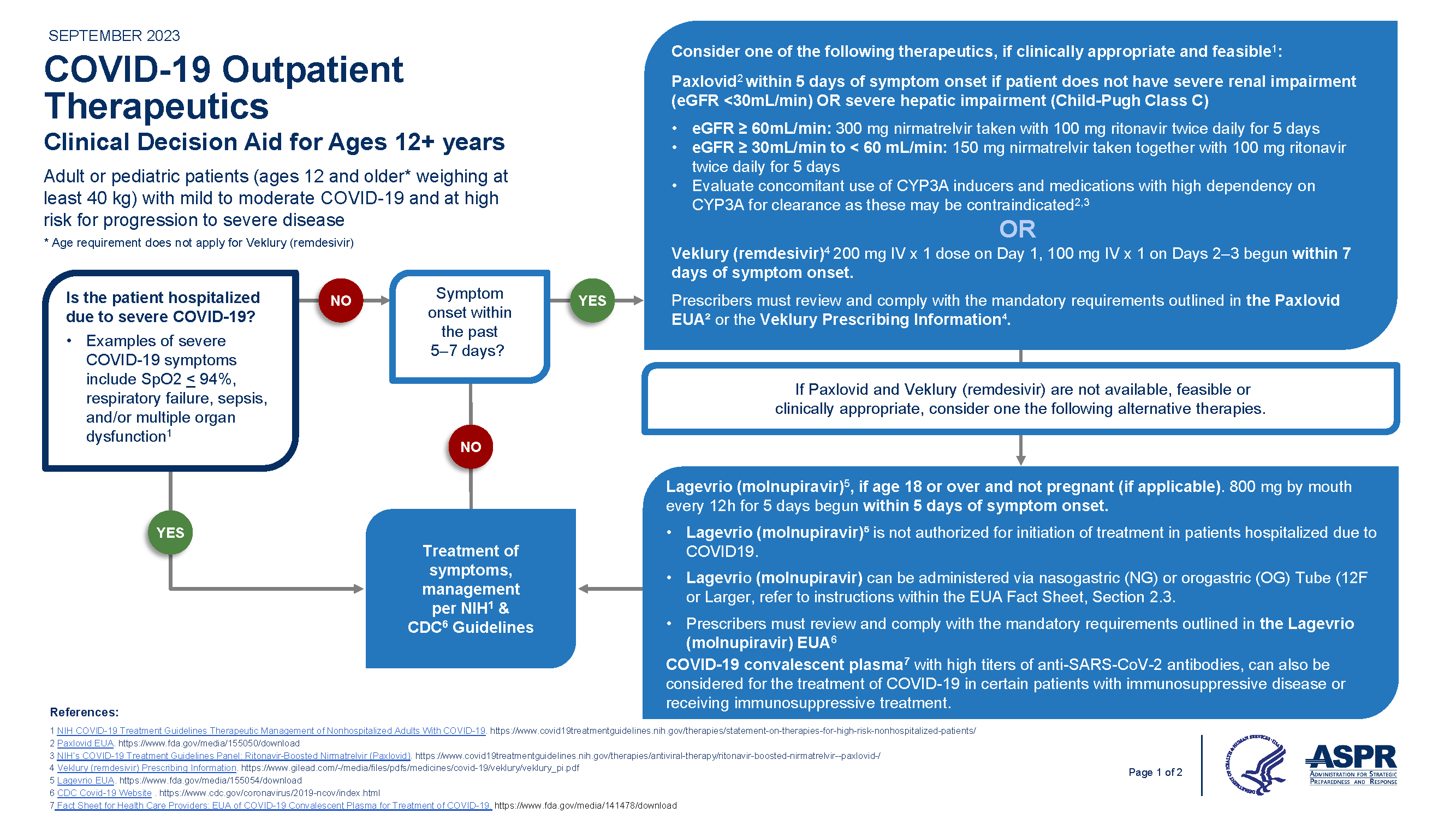
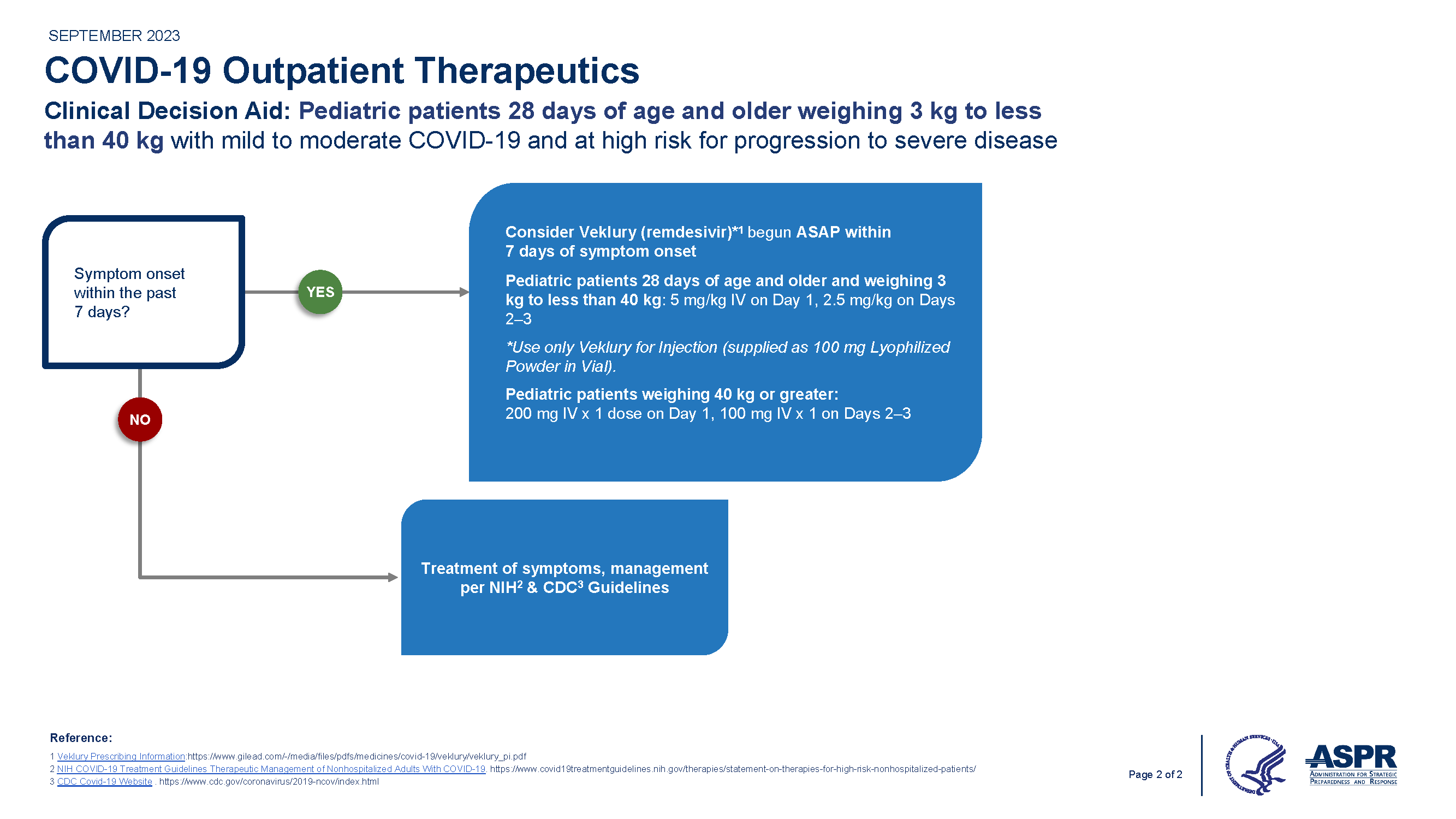
Treatment
*Adapted from COCA call 1/22/22
Clinicians should refer to the EUA healthcare provider fact sheets for further details on paxlovid, remdesivir, and molnupiravir. For the FDA-approved remdesivir (Veklury) for 12 yrs and older weight at least 40kg, refer to the full prescribing information.
Clinicians should refer to the EUA healthcare provider fact sheets for further details on paxlovid, remdesivir, and molnupiravir. For the FDA-approved remdesivir (Veklury) for 12 yrs and older weight at least 40kg, refer to the full prescribing information.
Prophylaxis
There are currently no authorized prophylactic treatments for COVID-19.
|
|
|
|---|---|
| Description |
|
| Patient Profiles |
|
Locate COVID-19 Therapeutics In The City
Patients and health care providers may use the ASPR Treatments Locator to search for sites with commercial COVID-19 therapeutics inventory. Locations participating in the Paxlovid USG Patient Assistance Program (PAP) are also included on the locator. Find pharmacies, clinics, and other locations with safe and effective low cost COVID-19 medications.
State and Federal Reporting Requirements
- Providers are expected to report all USG courses that they received and should report inventory, administration/dispensing, and disposal data until the provider’s USG product inventory is fully reconciled.
- Note: As of March 8, 2024, EUA-labeled Paxlovid is no longer authorized for use. All remaining EUA-labeled Paxlovid, regardless of expiration date, must be disposed of on site in accordance with all federal, state, and local regulations or returned for disposal.
- The U.S. Government has developed a mechanism for providers to report commercial treatment location data voluntarily that is now visible in an expanded treatments locator tool.
- Sites can voluntarily upload commercially acquired inventory data for Paxlovid, Lagevrio, and outpatient Veklury via HPOP. HPOP users can leverage their existing accounts to note product availability, while non-HPOP users can use the HPOP volunteer reporting website
- Sites only need to note that the product is available – not the number of treatment courses – once every 60 days, although more frequent updates provide more accurate information to patients and providers.
Provider Resource Library and Other Useful Links
- Webinars
- Research
- MMWR: No Association Found Between Virologic Rebound and Paxlovid Treatment
- MMWR: Information for Persons Who Are Immunocompromised Regarding Prevention and Treatment of SARS-CoV-2 Infection in the Context of Currently Circulating Omicron Sublineages
- MMWR: Summary of Guidance for Minimizing the Impact of COVID-19 on Individual Persons, Communities and Healthcare Systems
- CDC MMWR: Hospitalization and ED Encounters for COVID-19 After Paxlovid Treatment
- MMWR: Equitable Dispensing of COVID-19 Oral Antivirals
- MMWR: Racial and Ethnic Disparities in Outpatient Treatment of COVID-19
- MMWR: Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19–Associated Hospitalization Among Immunocompetent Adults Aged ≥65 Years
- Educational Tools
- Side-by-Side Overview of COVID-19 Therapeutics Authorized or Approved
- NIH COVID-19 Treatment Guidelines
- COVID-19 Treatment Guidelines and Considerations for the Immunocompromised
- Clinical Considerations for COVID-19 Treatment in Outpatients
- ASPR Paxlovid Info Sheet
- Paxlovid Drug interactions
- FDA Updated Paxlovid Fact Sheet
- NIH Paxlovid Checklist Tool for Prescribers
- FDA Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers
- FDA Updated Lagevrio Fact Sheet
- Lagevrio Information Sheet
- Veklury (remdesivir) Prescribing Information
- COVID-19 Drug Interactions Checker App
- Underlying Medical Conditions Associated With Higher Risk for Severe Covid-19
- Paxlovid FDA Letter of Authorization, updated November 1, 2023
- Printable Posters
- Other Links
Shelf life Extensions
Shelf-Life Extension infomation. Click the links to view the affected lot numbers.
New! Searchable database of COVID-19 Therapeutic Product Expiration Dates is now available
New! Searchable database of COVID-19 Therapeutic Product Expiration Dates is now available
- Evusheld
- Paxlovid
- Lagevrio
- Bebtelovimab
- Sotrovimab
- Guidelines for Product Return: Bamlanivimab, BAM/ETE, REGEN-COV, Sotrovimab
Access COVID-19 Antivirals for Free or at a Reduced Cost
On Nov. 1, 2023, Paxlovid and Lagevrio became commercially available. Patient assistance programs that help pay for these drugs are available to people who are underinsured, uninsured, or publicly insured through Medicaid, Medicare or other programs. See Sample Antiviral Access Programs
On Nov. 1, 2023, Paxlovid and Lagevrio became commercially available. Patient assistance programs that help pay for these drugs are available to people who are underinsured, uninsured, or publicly insured through Medicaid, Medicare or other programs. See Sample Antiviral Access Programs
