Become a Vax Provider - HAN
Jump To:
Interested in becoming a COVID-19 Vaccine Provider?
Please note: Completion of COVID-19 vaccine enrollment does not guarantee a facility will receive COVID-19 vaccine. Doses will be allocated based on vaccine availability, vaccine storage capacity, and facilities serving priority groups as defined by the National Academies of Sciences, Engineering and Medicine and the Advisory Committee on Immunization Practice
STEP 1: Review the CDC Program Provider Agreement and start collecting enrollment data
Before your facility submits enrollment materials in I-CARE, review and complete the CDC COVID-19 Vaccination Program Provider Agreement which is a PDF fillable form. This document should be completed electronically, including signatures, for easy uploading into I-CARE. The CDC COVID-19 Vaccination Program Provider Agreement contains two sections:
- Section A. COVID-19 Vaccination Program Provider Requirements and Legal Agreement
- Captures basic organizational, Chief Medical Officer (CMO), and Chief Executive Officer (CEO) information
- Outlines the agreement requirements
- Must be signed by the CMO and CEO
- Section B. COVID-19 Vaccination Program Provider Profile
- Captures site specific information such as vaccine shipping address, vaccine coordinator information and patient populations
- Facilities will need to include all prescribers (and medical license numbers) associated with their facility who may write orders for COVID-19 vaccine. Large health systems should think through which providers will fall into this role and do their best to include identified providers.
- Must be signed by the pharmacist, medical director, or vaccine coordinator.
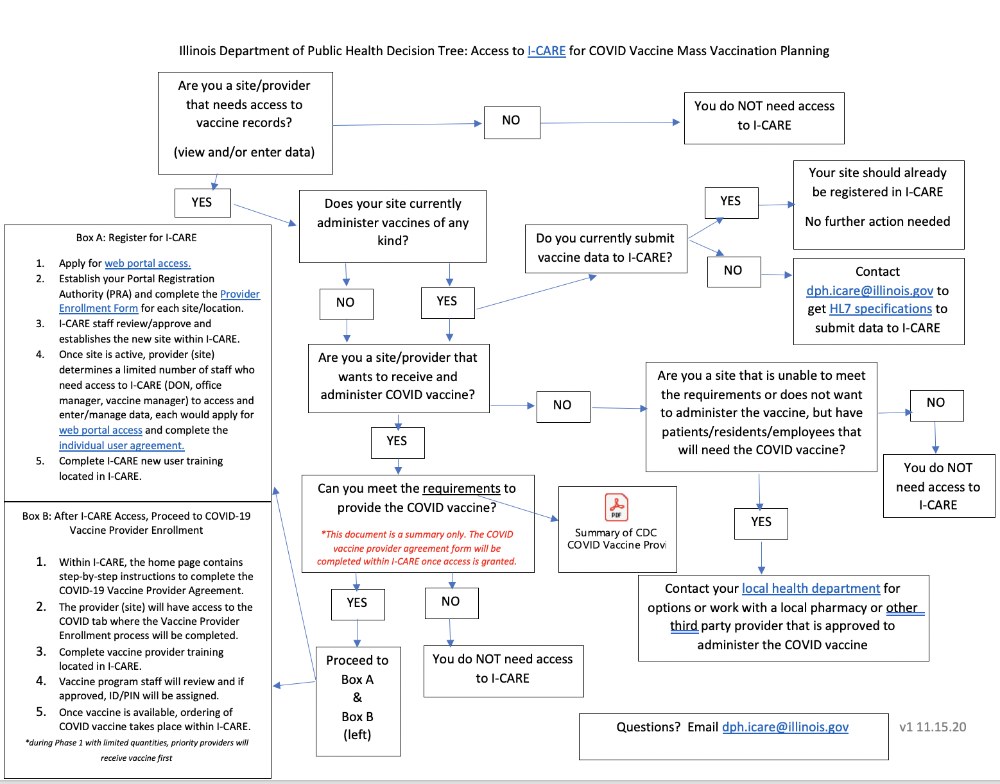
STEP 2: Ensure your facility is enrolled in I-CARE and all necessary staff have I-CARE Accounts
As outlined in the Program Agreement, all COVID-19 vaccine doses must be reported to the Illinois immunization information system (I-CARE) within 24 hours of administration. This includes doses entered via manual data entry into I-CARE and those electronically sent through an Electronic Health Record (EHR) system or through another mechanism.
As part of the planning process, each site administering COVID-19 vaccine should:
- Ensure the facility is enrolled in I-CARE
- Verify that your EMR/EHR is set-up to document COVID-19 doses and whether it can send doses to I-CARE automatically via HL7
- If you are not yet setup for reporting via HL7 you will need to setup your connection by emailing IDPH at: DPH.HL7ICARE@illinois.gov or establish a process for manual entry of doses into I-CARE
- Establish a process for recalling/reminding patients due for the second dose of COVID-19 vaccine
Facilities that need to enroll in I-CARE, can find the I-CARE Provider Site Enrollment form here.
Requesting I-CARE access for individuals
At least two individuals per facility must have their own unique login ID and password. A good place to start is ensuring the COVID-19 primary and back-up vaccine coordinators listed on the CDC COVID-19 Vaccination Program Provider Agreement have I-CARE access. Access for new users can be requested by submitting an Individual Agreement and applying for a web portal account: https://wpur.dph.illinois.gov/WPUR/.
I-CARE Training
I-CARE users may access a variety of training tools by selecting the new user button on the I-CARE application home page. These training tools include:
- The I-CARE user manual
- “How To” Training Videos
- Tips and Tricks
- What’s New
STEP 3: Ensure vaccine storage and handling and become familar with the CDC Toolkit
Ensure storage units are working well, have adequate storage space and temperatures are being monitored 24 hours a day and recorded twice daily.
There are three different vaccine cold chain parameters (depending on product):
- Refrigerated (2-8C)
- Frozen (-20C)
- Ultracold (-80C)
Designate a minimum of two employees who will be trained to receive deliveries of COVID-19 vaccine and are available to receive phone calls from delivery personnel. These individuals will be designated as primary and back-up vaccine coordinators. Ensure staff review CDC’s Vaccine Storage and Handling Toolkit.
CDPH will disseminate COVID-19 vaccine specific storage and handling guidance when it becomes available.
Provider Contacts
COVID-19 Vaccine Contact Information
Please direct all questions to COVID19vaccine@cityofchicago.org
For Information On The Vaccine Please Go To:
www.chicago.gov/covidvax
Please visit the Illinois Department of Public Health I-CARE website for information on enrolling a facility or individual in I-CARE: https://www.dph.illinois.gov/topics-services/prevention-wellness/immunization/icare